Which One of the Following Is Not an Intensive Property
For example boiling point density color melting point Odor temperature etc. Complete step by step answer.
Difference Between Intensive And Extensive Properties Definition Examples And Differences
According to the definitions density pressure and temperature are intensive properties and volume internal energy are extensive properties.

. Some common intensive properties are temperature density hardness of a substance etc. Which of the following is an intensive property. A mass B temperature C heat content D volume E amount.
See the answer See the answer See the answer done loading. Which one of the following is NOT an intensive property. An intensive property is a physical property of a system that does not depend on the system size or the amount of material in the system.
Light light 5 out of 5 points 5 out of 5 points 5 out of 5 points 5 out of 5 points 5 out of 5 points 5 out of 5 points. 4 rows The temperature of a body does not depend on the amount of mass of a substance. Which one has more energy.
A large pot with an extensive property. Pressure temperature density specific heat surface tension viscosity molar volume etc. Pressure and molar volume C.
Among the following the intensive property is properties are. Mass crosses the boundary but not the energy E. Add your answer and earn points.
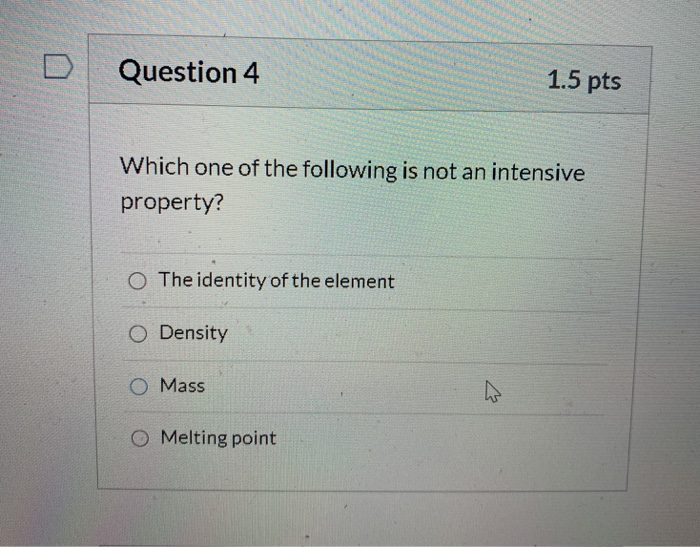
10th - 12th grade. Which one of the following is not an intensive property. A density B temperature C melting point D mass E boiling point.
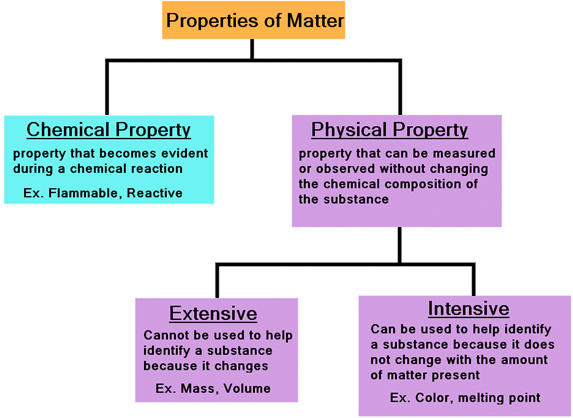
The properties of the system which depend only on the nature of matter but not on the quantity of matter are called intensive properties. Temperature and density B. Which one of the following is not and intensive property A density B temperature C melting point D mass E boiling point.
Heat capacity and enthalpy are extensive properties since they depend upon the quantity of matter in the system. Melting point of 735. Temperature and pressure belongs to intensive properties.
It is a physical property of that system. Of the following only what is an extensive property A density B volume. A cup with an intensive property.
Properties of Matter DRAFT. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount. The properties whose value does not depend on the quantity of matter present in the system are known as intensive properties.
A large pot with an intensive property. Both are the same with an intensive property. Mass does not cross boundaries of the system though energy may do so B.
Mass Mass Question 14 Selected Answer. Both energy and mass cross the boundaries of the system D. One of the following is not a physical property of iron.
A density B temperature C melting point D mass E boiling point of the following is the highest temperature. Which one of the following is an intensive property and whyMassTemperature AmountVolume. Neither mass nor energy crosses the boundaries of the system C.
Which of the following is not an intensive physical property. If gas has say. A Pressure b Temperature c Density d Heat.
The properties which do not depend upon the quantity of matter present in the system or size of the system are called intensive properties. An isolated system is one in which_____. Pressure specific heat temperature density etc.
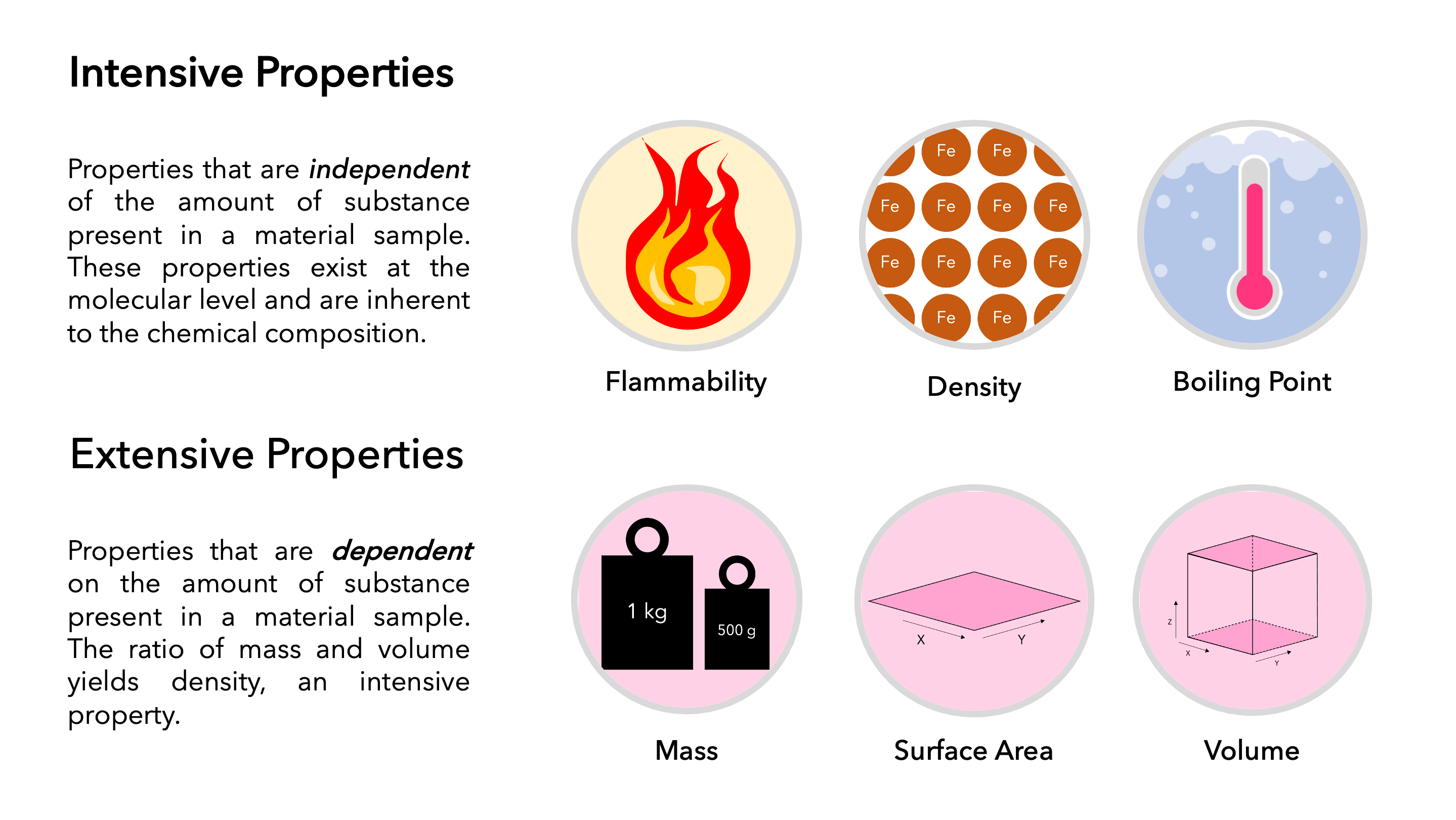
-A property is intensive if it does not depend on the size of the system or amount of material in the system. Which of the following is not the intensive property. Which one of the following is an extensive property.
Which one of the following is an intensive property. Which one of the following is not an intensive property. A density B temperature C melting point D mass E boiling point.
Chemistry questions and answers. Molar conductivity and electromotive force emf are intensive properties as these are size independent. Molarity is the of the following is not an extensive property and molarity is the concentration of the solution and expressed as the number of moles of solute per litre of solution.
Which one of the following pairs does not represent example for intensive property. A Molar volume b Density c Molaritv d Entropy asked Oct 9 2020 in Thermodynamics by Manish01 476k points. An intensive property is a type of physical property in which the value do not depend upon the amount of substance.
It is a bulk property that does not depend on the size of the matter or that of the system. Which is an intensive property. Heat capacity and enthalpy.
A cup with an extensive property. Which one of the following is an intensive property and why. Molarity mole of solute liter of solution.
Volume 1 See answer Advertisement Advertisement sansca9552 is waiting for your help. Molar heat capacity and density D. This problem has been solved.
Here volume depends on the quantity of matter. You have a sample of cobalt chloride with the following properties. Therefore volume is not an intensive property.
Density of 3356 gcm 3. Which one of the following is an intensive property A mass B temperature C length D volume E amount. In the following list only __________ is are NOT an example of matter.
Thermodynamic reactions do not occur. - A property is extensive if it depends on the size of the system as well as amount of the material in the system.
Intensive Vs Extensive Properties Psiberg

Density An Intensive Property What Are Extensive And Intensive Properties Property Refers To The Characteristics Of A Material Property Refers To Ppt Download

Intensive Property Easy Science Chemistry Experiments Education Subjects Easy Science

Do You Know The Difference Between Intensive And Extensive Properties Properties Of Matter Physical Properties Of Matter Intense
Properties Extensive And Intensive Texas Gateway

Solved Which One Of The Following Is Not An Intensive Chegg Com
Intensive Vs Extensive Properties Psiberg
Intensive Vs Extensive Properties Psiberg
Extensive Vs Intensive Properties Overview Examples Expii

Which Of The Following Is Not An Intensive Property Youtube

Intensive Properties Extensive Properties Details In 2021 Intense Internal Energy Potential Energy

Thermochemistry Mcqs Pin 2 Chemistry Paper Chemistry State Function

Properties Of Matter Chemistry Basics Chemistry Classroom Physical Science High School

In Thermodynamics Which One Of The Following Properties Is Not An Intensive Property

Solved 12 Which One Of The Following Properties Does Not Chegg Com
Solved Question 4 1 5 Pts Which One Of The Following Is Not Chegg Com
Intensive Vs Extensive Properties Psiberg
Solved 7 Which One Of The Following Is Not An Intensive Property Course Hero

Difference Between Intensive And Extensive Properties Of Matter Properties Of Matter Intense Physical Properties Of Matter
Comments
Post a Comment